From asymptomatic roots, avirulent Fusarium isolates can be isolated.
INDUCED SYSTEMIC RESISTANCE (ISR): Induced systemic resistance has been identified as main mode of action in many of the non-pathogenic strains of F. oxysporum. First reported by Biles and Martyn (1989) ISR has been reported by many workers....
ANTIBIOSIS: ... Hua et al. (2015) observed the non-pathogenic Fusarium isolate CanR46 could produce anti-fungal compounds 5-hexenoic acid, limonene, octanoic acid and 3,4-2H-dihydropyran that inhibited mycelium growth, germination of conidia and germ-tube elongation in Verticillium dahliae and prevented wilt in cotton.... Rodriguez et al. (2006) reported another anti-fungal compound cyclosporine. It was produced by non-pathogenic Fusarium strain S6A inhibited the sclerotia formation in Sclerotinia sclerotiorum infecting soybean. Other compounds produced by non-infective isolate include saponins, phenol, flavonoid, tannins, alkaloids, anthroquinons and terpenoids in culture filtrates and some of them are toxic to fungi and bacteria (Nawar 2016).
ROOT COLONIZATION: ... Alterations in composition of root exudates due to non-pathogenic Fusaria was observed by Schouten et al. (2009) that reduced the nematode attraction and resulted in repellence to nematodes.
PLANT GROWTH PROMOTION (PGP): Besides inducing systemic resistance, these isolates also have ability to promote plant growth as observed by Pocasangre (2000) in banana. Treatment with non-pathogenic Fusarium isolates improved the tomato plant growth parameters viz., length and weight of root and shoot (Patil et al. 2011b). All these non-pathogenic Fusarium cultures were positive for phosphate solubilisation and production of IAA and GA....
MOLECULAR CHARACTERIZATION OF NON-PATHOGENIC FUSARIUM: The sequence analysis with ITS region of the non-pathogenic Fusarium cultures along with ITS sequence of standard pathogenic isolates revealed that the Fusarium isolates viz., F. moniliforme, F. oxysporum, F. solani and F. merismoides that did not infect tomato were distinct from not only the tomato pathogen, F. oxysporum, f. sp. lycopersici, but from all the other pathogenic Fusarium of banana, chilli, red gram, bengal gram, cucurbit, etc. (Patil 2009).... Kurtz et al. (2009) used RFLP in the IGS region and found that non-pathogenic isolates of F. oxysporum f. sp. cubense were similar irrespective of their diverse geographical location.
EFFECT OF ENVIRONMENTAL CONDITIONS ON EFFICACY OF NON-PATHOGENIC FUSARIUM: Larkin and Fravel (2002) found that these isolates differed in their efficacy in different soil types. Isolate CS1 reduced wilt severity in tomato in sandy and loamy soil and not in clay soil. Isolate CS20 was effective at various environmental conditions. Non-pathogenic isolates differed in their performance at diverse temperature regime....
POSSIBILITY OF NON-PATHOGENIC ISOLATES TURNING INTO PATHOGENIC ISOLATES: Ma et al. (2010) experimentally proved that a non-pathogenic Fusarium species could become pathogenic on tomato by horizontal chromosome transfer (HCT) by transferring a pathogenicity chromosome from Fusarium oxysporum f. sp. lycopersici that causes tomato wilt. This transfer of lineage-specific chromosome between genetically distant strains indicated the polyphyletic origin of host specificity genes in Fusarium species. These lineage-specific chromosomes were rich in transposons and contained genes related to signal transduction and effector proteins related to pathogenicity and virulence. It was also hypothesized that chromosome 14 from F. oxysporum f. sp. lycopersici was responsible for pathogenicity, and transfer of this chromosome can convert the non-pathogenic strains to pathogenic to tomato. This chromosome 14 contained effector genes viz. SIX1, SIX2, SIX3, SIX5, SIX6 and SIX7, which are responsible for virulence of the pathogen.
• 2021 - "An Ecological Insight into the Multifaceted World of Plant-Endophyte Association", by Sushma Mishra et al., Critical Reviews in Plant Sciences.
EXCERPTS: ... Based on the phylogenetic affiliation, metabolic potential, physiological features, and mode of transmission, endophytes can be classified into systemic and nonsystemic endophytes (Wani et al., 2015). Systemic endophytes, also known as true endophytes, represent the typical class of endophytes that exist in a symbiotic relationship with the host plant, and do not cause disease at any stage of the life cycle. The systemic endophytes are also co-cladogenetic, i.e. the plant may host the same set of endophytes irrespective of environmental conditions (Botella and Diez, 2011; Higgins et al., 2014). Nonsystemic endophytes, on the other hand, are the microorganisms that live asymptomatically within the plant tissues for at least a part of their life cycle. Such associations are typically short-lived and seasonal; their diversity varies with changes in the host's physiological parameters and varying environmental conditions (Botella and Diez, 2011). Another striking feature of nonsystemic endophytes is that they may turn pathogenic when the host plant is stressed or resource-limited (Petrini, 1991).
A plant may attain its microbiota either through horizontal transmission, i.e. from the environment (mainly rhizosphere or phyllosphere), and/or vertical transmission, i.e. from one generation to the next, through seeds or vegetative propagules (Frank et al., 2017; Kumar et al., 2020).
In another study, the same microbe Fusarium verticillioides, demonstrated two modes of lifestyle in maize: pathogenic and endophytic, depending upon the environmental factors and the host genotype (Bacon et al., 2008). The authors reported that the fungus switches to its pathogenic state when the plant is stressed.... It needs to be emphasized that the boundaries between the three types of symbiotic associations (mutualism, commensalism, and parasitism) are not well demarcated. Depending on many factors including environmental conditions, biotic and abiotic stresses, and microbial interactions, the equation between the plant and microbe changes.
• 2021 - "Endophytic fungi: a tool for plant growth
promotion and sustainable agriculture", by Noemi Carla Baron & Everlon Cid Rigobelo, Mycology.
EXCERPTS: The association between plants and fungi is extremely common. Fossil records indicate that the existence of this union with endophytes and mycorrhizas have existed for more than 400 million years (Krings et al. 2007; Chadha et al. 2015) starting when plants colonised the soil, thus indicating the importance of this group in the evolution of this process (Rodriguez et al. 2009; Rai et al. 2014; Anjum et al. 2019). The positive aspects of this interaction have always been noted and discussed, but in-depth studies evaluating the real benefit provided by these fungi have only been performed recently (Busby et al. 2016; Card et al. 2016; Vega 2018; Quesada-Moraga 2020).
... The endophytic interaction is defined as balanced antagonism (Schulz et al. 2015) because the recognition of the plant as a host requires the activation of virulence mechanisms for colonisation and the triggering of host defences by these events. While an equilibrium exists in this interaction, the fungus survives of nutrients from the host plant and, in exchange, provides benefits, including tolerance to biotic and abiotic stresses (Bamisile et al. 2018).... Fungi are able to act as antagonists of plant pathogens through the use of a diverse range of mechanisms, such as the production of metabolites (antibiotics, volatile compounds and enzymes), engagement in competition (for space, carbon sources, nitrogen and minerals) and parasitism, induction of systemic resistance by the plant and increases in plant growth, resulting in the reduction of the activity of the pathogens (Vega et al. 2009; Vidal and Jaber 2015; Vega 2018; Lr 2018; Quesada- Moraga 2020).
... Some fungal endophytes are able to colonise a wide range of plant species, while others are more specific and occur only inside a restricted number of plants. Additionally, specificity can also be present in relation to the portion of the plant that is colonised (Aly et al. 2011; Bamisile et al. 2018). Apparently, vertically transmitted fungi seem to present plant associations with a more mutualistic profile than horizontally transmitted fungi, which are more likely antagonists (Aly et al. 2011).... Horizontal transmission occurs when vegetative propagules or spores are produced by the endophyte and spread to the plant population through the air or via some vector, while vertical transmission consists of the transference of the fungi to the plant progeny via seeds.
... The genetic mechanisms involved in stress tolerance are poorly known, and an essential aspect is not considered in this process: the symbiotic association of plants and microorganisms (Chadha et al. 2015). The fungal endophyte community that exists in wild plants can be severely modified, and many representatives can be lost during domestication; therefore, fungi are harmed by losing their safe niche and plants are deprived of a partnership that could improve their ability to overcome environmental challenges (Lugtenberg et al. 2016). For a deeper discussion about the reasons why endophytes can be lost during plant breeding, see Lugtenberg et al. (2016). For endophytes, the inner part of the plant is a protected niche that contains the necessary nutrients for fungal survival and growth in addition to presenting low competition with other microorganisms. Therefore, in exchange for this safe place, fungi improve plant fitness by several mechanisms (Khan et al. 2015; Lugtenberg et al. 2016; Chitnis et al. 2020). The benefits of plant colonisation by endophytic fungi can occur directly and/or indirectly, and the differentiation among them is complex (Berg 2009). Among the direct mechanisms of growth promotion, the most important are the acquisition of nutrients and the production of phytohormones, while tolerance to biotic and abiotic stresses, including combat against pathogens, is considered an indirect aspect in the promotion of growth (Hardoim et al. 2015; Souza and dos Santos 2017).
Production of phytohormones: Endophytic fungi are able to produce auxins, gibberellins (GAs) and cytokinins. The potential of phytohormone production by endophytic fungi is underexplored, especially for gibberellins, even though these molecules are as important as chemical signalling and messengers for plant growth in different environmental conditions (Khan et al. 2015).... Gibberellins are essential in several plant responses, including seed germination, stem elongation, sexual expression, flourishing, fruit formation and senescence (Bomke and Tudzynski 2009). The production of gibberellins by endophytic fungi is described as occurring from acetyl-CoA by the mevalonic acid (MVA) pathway....
Activation of systemic resistance: Endophytic fungi can aid plants in improving their self-defence system, thus promoting the activation of induced systemic resistance (ISR) pathways, which may overlap with that of acquired systemic resistance (ASR) because both systems can improve plant growth (Berg 2009; Busby et al. 2016) and protect against pests and pathogens (Chadha et al. 2015).... The balanced interaction between fungal endophytes and their plant hosts occurs due to the lack of pathogenic properties. A good example is the comparison of Brassicaceae's endophytic strain Colletotrichum tofieldiae and the pathogenic Colletotrichum incanum in Arabidopsis thaliana. Evolution has negatively selected genes of effector proteins in the endophytic strain, which are directly involved in the pathogenic action at the moment of plant colonisation, and the same did not occur with the pathogenic strain C. incanum.
Production of antibiotics and secondary metabolites: In addition to stimulating the production of defence molecules by the plant itself, endophytic fungi are a large reservoir of molecules that act in favour of their host. They are excellent producers of compounds with activity against pathogens and herbivores, including alkaloids, steroids, terpenoids, peptides, polyketones, flavonoids, quinols, phenols, chlorinated compounds and volatile organic compounds (VOCs) (Card et al. 2016; Lugtenberg et al. 2016; Latz et al. 2018; Kaddes et al. 2019). Moreover, studies report the production of compounds with antiviral, antibacterial, antifungal and insect action (Card et al. 2016; Latz et al. 2018).... An uncountable number of molecules are produced as secondary metabolites by endophytic fungi; however, specific pathways and substances have not been well characterised thus far. The review of Lugtenberg et al. (2016) is recommended for deeper knowledge of the chemical structures of some secondary metabolites produced by endophytic fungi.... Among the wide range of secondary metabolites produced by endophytic fungi, more than 300 of these molecules are VOCs (Lugtenberg et al. 2016; Kaddes et al. 2019). They consist of small molecules, presenting high vapour pressure, and they are easily diffusible through the cell membrane, in the atmosphere and in the soil, which makes them special agents of fungal communication with other organisms, including plants, in addition to presenting bioactivity against many pathogens (Kaddes et al. 2019).
Protection against biotic and abiotic stresses: Environmental degradation by agricultural processes and global climate change expose plants to increasingly challenging conditions for their growth and maintenance.... Endophytic fungi are able to combat abiotic stresses, including drought, high and low temperatures, salinity and toxic heavy metals (Aly et al. 2011; Khan et al. 2015). For biotic stress protection, fungal endophytes are responsible for the activation of ISR and ASR, which produce metabolites against pathogens; moreover, parasitism or competition can occur to avoid disease and herbivory (Chadha et al. 2015; Chitnis et al. 2020).... Endophytic fungi help host plants adapt to stress conditions through diverse mechanisms. As reviewed by Khan et al. (2015) and Yan et al. (2019), during oxidative stress, plants increase the activity of antioxidant enzymes, mainly catalases and peroxidases, which leads to the production of ROS, resulting in membrane attack causing the peroxidation of membrane lipids. By some not yet defined mechanisms, endophytic fungi confer tolerance to ROS, reducing lipid peroxidation. Another important problem caused by abiotic stresses (drought, heat and salinity) in membranes is electrolyte leakage, which is associated with the variation in the lipidic composition and the amount of these molecules of the cell membrane due to stress conditions. Endophytic fungi are able to induce changes in the lipidic composition of the cell membrane, preventing leakage (Khan et al. 2015; Yan et al. 2019).
... In relation to biotic stress, the main defences against pathogens, herbivores and nematodes are the production of secondary metabolites and the activation of systemic resistance by endophytic fungi (Latz et al. 2018; Yan et al. 2019; Poveda et al. 2020).
• 2021 - "Protection to Tomato Wilt Disease Conferred by the Nonpathogen Fusarium oxysporum Fo47 Is More Effective Than that Conferred by Avirulent Strains", by Francisco J. de Lamo et al., Phytopathology.
ABSTRACT EXCERPT: Although the vascular pathogen Fusarium oxysporum is notorious for being the causal agent of Fusarium wilt disease, the vast majority of F. oxysporum strains are harmless soil and root colonizers. The latter F. oxysporum's are often endophytes colonizing roots intracellularly without negatively affecting plant fitness. Actually, most of them, like Fo47, are beneficial providing biological control to various root pathogens.
EXCERPTS: Fusarium oxysporum, a filamentous ascomycete, is the causal agent of Fusarium wilt disease (Michielse and Rep 2009). This soilborne vascular fungus ranks among the top 10 major fungal plant pathogens (Dean et al. 2012). Each F. oxysporum pathogen harbors a specific set of effector genes required for pathogenicity on a specific host (van Dam et al. 2016). Nevertheless, the ample majority of F. oxysporum strains are nonpathogenic saprotrophs able to endophytically colonize plant roots (Bao et al. 2004). It has been well established that wilt disease-suppressive soils contain beneficial F. oxysporum endophytes that are responsible for protecting a susceptible host to pathogenic F. oxysporum strains (Alabouvette 1986; Tamietti et al. 1993). These F. oxysporum endophytes exert biological control by directly affecting the invading pathogen and/or by inducing plant immune responses.... In summary, compared with the endophyte Fo47, avirulent pathogens only weakly reduce susceptibility to F. oxysporum f. sp. lycopersici. A possible explanation for this observation is that the Fo47 endophyte is less, or even unable to suppress host immune responses. Presumably, once immune responses are triggered by an endophyte they cannot be suppressed by the effectors present in a virulent strain, or it is to a much lower extent not able to significantly contribute to resistance.
• Note by Connie Barlow: Thankfully, the genus Fusarium has pathogenic strains that so widely evoke disease symptoms in crop plants that funding has been available to discern both the geographic span and the diversity of effects on host plants — ranging from beneficial to asymptomatic to pathogenic — that distinct strains of the same Fusarium species can evoke. For those of us grounded in organismic or ecological aspects of the biological sciences, this ability of a known fungal pathogen to also express beneficially in a plant is surprising. Hence, the distinguishing and naming of a new Fusarium species (distinct from well-known F. Lateritium) and as later described as "the primary cause of Florida torreya decline" (Dreaden et al. 2020) evoked a great deal of fear of disease transmittal into other native trees, such that seed distributions from the ex situ official orchards in north Georgia were severely diminished. It is therefore my hope that botanical garden and Native Plant Society staff (especially in Georgia and Florida) will familiarize themselves with the broader scientific literature on the Fusarium genus, such as evident in this paper.
• 2021 - REVIEW: "Endophytic Fungi: From Symbiosis to Secondary Metabolite Communications or Vice Versa?", by Beena Alam et al., Frontiers in Plant Science.
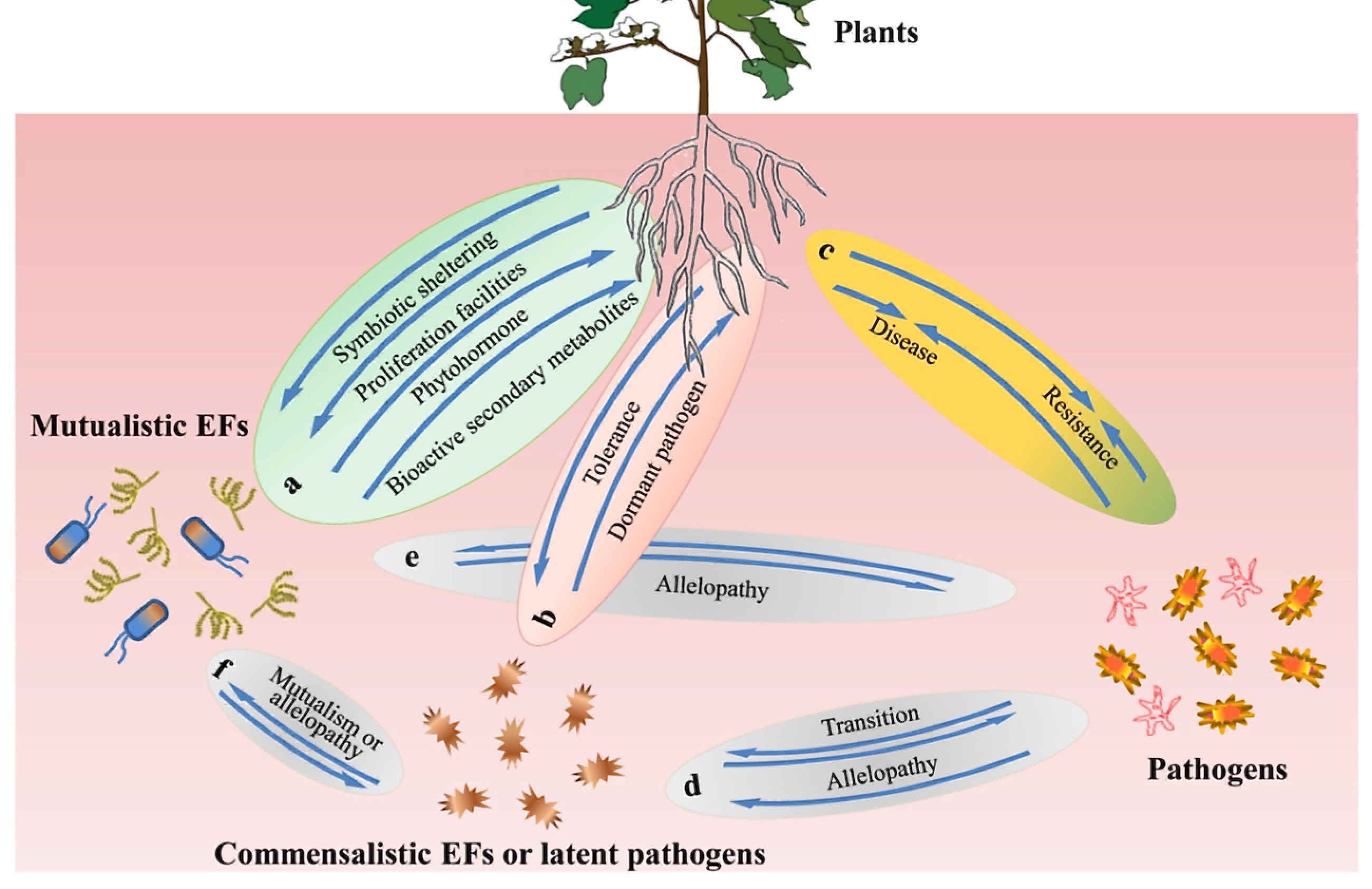
ABOVE: "EF" = Endophytic Fungi. "During infection, fungi form three corresponding types of interactions with hosts: mutualistic (beneficial endophytes), commensalistic (non-beneficial/virulent endophytes), and pathogenic (virulent pathogens), depending on the physiological status or specific circumstances that host plants experience. According to these three modes of action, fungal strains can increase, have no palpable effects on, or decrease host fitness (Kogel et al., 2006).
EXCERPTS: ... According to their colonizing behaviors, endophytic microflora can be sorted into facultative and obligate categories. Facultative endophytes colonize plants at certain stages of their life cycles, but they may also reside outside the plant at other stages to form an association with the immediate rhizosphere soil of host plants (Abreu-Tarazi et al., 2010). In contrast, obligate strains live in plants throughout their entire life cycles. They usually proliferate across plant generations through vertical transmission and use or alter the metabolic machinery and products of plants for their own survival (Hardoim et al., 2008; Gouda et al., 2016).
... Among these endophytic microorganisms, endophytic fungi (EFs) have attracted much research interest because they have provided not only novel sources of cytotoxic compounds, such as anticarcinogenic molecules (Uzma et al., 2018) and antibacterial substances (Radic and Strukelj, 2012), but also biostimulants for essential oil biosynthesis (El Enshasy et al., 2019). They may enhance nutrient solubilization in the plant rhizosphere (Mehta et al., 2019), promote plant growth (Poveda et al., 2021), act as biological control agents (Poveda and Baptista, 2021), or activate plant systemic resistances to biotic (Poveda et al., 2020a) or abiotic (Cui et al., 2021) stresses.
... According to the reproductive pattern and host occurrence, EF communities can be sorted into two categories: the Clavicipitaceous/Balansiaceous group (C-group) and the non-Clavicipitaceous/non-Balansiaceous group (NC-group).... Some illustrative endophytic mycobiomes of the NC-group include Fusarium spp., Piriformospora indica, and dark septate mycobiota (Varma et al., 2000; Schulz et al., 2002).
... Fungi that have been identified as endophytes that are also possible pathogens include Cladosporium, Fusarium, Colletotrichum, Cordana, Deightoniella, Periconiella, Verticillium, Curvularia, Nigrospora, Guignardia, and Phoma (Photita et al., 2004; Cui et al., 2021). These EFs stay in latent or dormant state in the tissue of their host plants long before the outbreak of disease symptoms. In such cases, the dormancy phase is essential because it determines the time when the fungus is harmless as an endophyte and when it is virulent as a pathogen. In the virulent phase, EFs cause obvious symptoms and change the morphology and physiology of host plants under adverse conditions (Figure 1d). It is precisely these hostile conditions, including malnutrition, disruption of ontogenetic state (Sieber, 2007; Rodriguez and Redman, 2008), biotic stresses, drastic climate changes
(such as elevated temperature and excessive humidity), and senescence, that break the balance between EFs and their hosts and lead to the transition of EFs from latent mode to active virulent pathogens, although there are no obvious disease symptoms before transition (Romero et al., 2001; Photita et al., 2004; Poveda et al., 2020b).